Scoop.it
Congressional Watchdog to Launch Probe into FDA Medical Device Recall Oversight
Posted on Leave a Comment

Two prominent senators called for an inquiry into the agency’s handling of device recalls through an open letter published last month.
Read the full article at: www.mddionline.com
Continue Reading »Cognito opens biomarker study of neuromod device for Alzheimer’s
Posted on Leave a Comment

As it races toward FDA review of its light- and sound-based neuromodulation device, Cognito Therapeutics is taking an even closer look at the tech.
Read the full article at: www.fiercebiotech.com
Continue Reading »FDA clears vibrating belt to boost brittle bones in women facing osteoporosis
Posted on Leave a Comment

The FDA has granted a clearance to its first prescription medical device to help treat low bone density, with a wearable vibrating belt designed for postmenopausal women.
Read the full article at: www.fiercebiotech.com
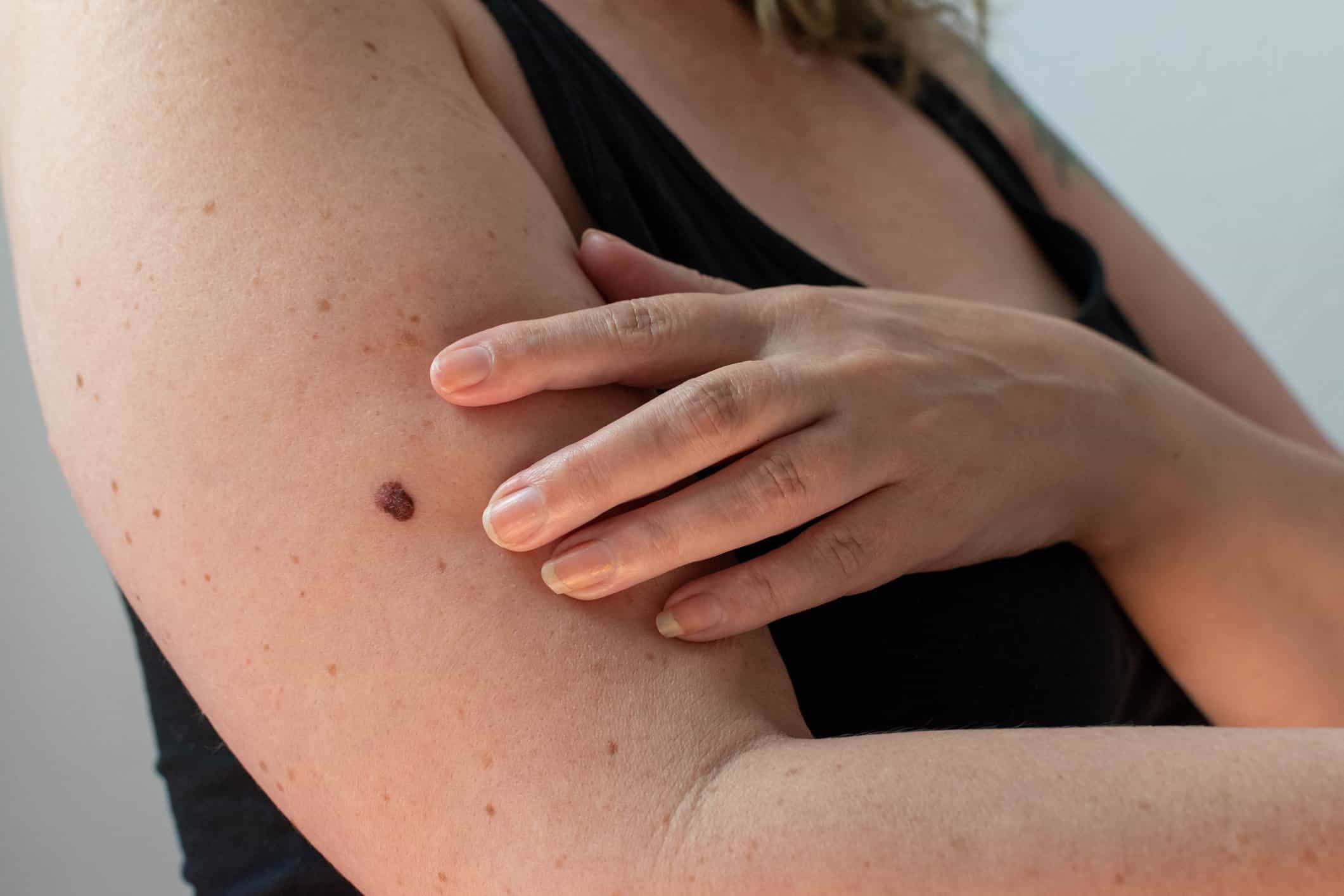
Continue Reading »FDA clears AI-powered handheld that checks moles for skin cancer
Posted on Leave a Comment
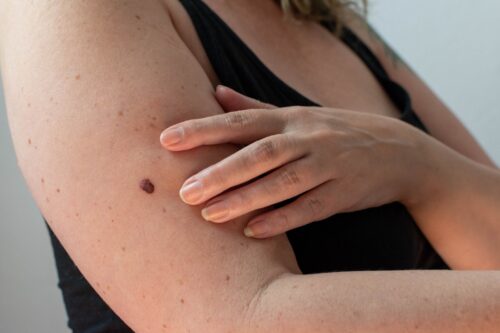
Designed for use by primary care providers, the noninvasive system can help identify melanoma, basal cell carcinoma and squamous cell carcinoma.
Read the full article at: www.fiercebiotech.com
Continue Reading »FDA, CMS issue joint letter supporting increased oversight of lab-developed tests
Posted on Leave a Comment

“CMS does not have the expertise to assure that tests work; the FDA does,” wrote leaders from both federal agencies.
Read the full article at: www.fiercebiotech.com
Continue Reading »FDA sets new record for novel device green lights in CDRH annual report
Posted on Leave a Comment

In what the FDA described as a “transformative year” for its Center for Devices and Radiological Health, the agency set a new record in issuing green lights to innovative medical devices and diagnostic tests, topping its history spanning more than four decades.
Read the full article at: www.fiercebiotech.com
Continue Reading »FDA OKs Neuralace’s nerve stimulation tech for diabetes pain
Posted on Leave a Comment
The original FDA nod for Neuralace’s Axon Therapy for nerve pain came after a decade of R&D. The road to its next regulatory nod was much shorter.
Read the full article at: www.fiercebiotech.com
Continue Reading »The increasing demand for microfluidics devices
Posted on Leave a Comment

Ultra-precision mass production for microfluidics devices,
Read the full article at: www.todaysmedicaldevelopments.com
Continue Reading »Artificial Intelligence, Part 2: Human Interaction, Liability, and Patient Safety
Posted on Leave a Comment

Listen to this in-depth discussion on how AI can help identify end-to-end data weaknesses, as well as broader implications regarding the inevitability of human interaction.
Read the full article at: www.biospace.com
Continue Reading »Signati™ Medical, Inc. Receives FDA Approval for IDE Study
Posted on Leave a Comment

Signati™ Medical, Inc.Receives FDA Approval for IDE Study – read this article along with other careers information, tips and advice on BioSpace…
Read the full article at: www.biospace.com
Continue Reading »