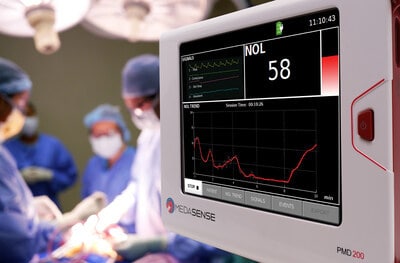
FDA authorizes marketing of Medasense’s NOL® (nociception level index) technology through a De Novo grant
FDA authorizes marketing of Medasense’s NOL® (nociception level index) technology through a De Novo grant – read this article along with other careers information, tips and advice on BioSpace…
Read the full article at: www.biospace.com
Continue Reading »Is an Extractables Study Appropriate for Limited-Contact Devices?

ISO 10993-1 is the cornerstone standard that is broadly recognized and accepted around the world, which is critical if you’re intending to market your device…
Read the full article at: www.mddionline.com
Continue Reading »Virtual Incision’s MIRA Completes FDA IDE Study

The miniaturized robotic-assisted surgery study will be used to support the company’s FDA De Novo request for market authorization.
Read the full article at: www.mddionline.com
Continue Reading »Supercapacitor Patch Could Solve Power Problem for Wearables

A MXene-based solution can integrate with fabric provide to energy for health-monitoring wearables devices.
Read the full article at: www.designnews.com
Continue Reading »Nasdaq delists Titan Medical amid mass layoffs, failed sale
After more than a year spent trying to coax its share price out of penny stock territory—to no avail—Titan Medical has been kicked off the Nasdaq.
Read the full article at: www.fiercebiotech.com
Continue Reading »Economic Constriction has Fallout on Investments, IPOs and Collaborations
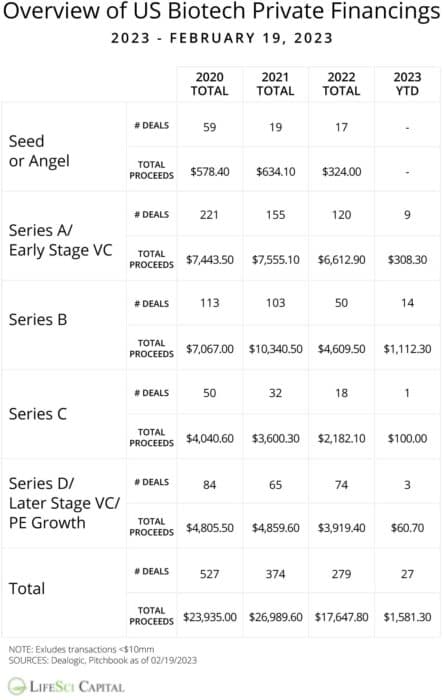
As the macro pressures of higher rates and fear of recession build, today’s investor is increasingly risk averse. With zero-risk options offering decent returns, only the highest-quality programs will get funding.
Read the full article at: www.biospace.com
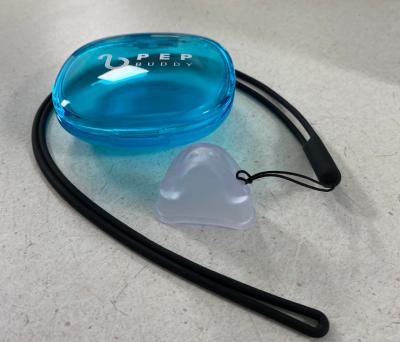
Continue Reading »Study sees COPD patients’ breathing boosted by hands-free device
A recent study found that startup PEP Buddy’s eponymous device significantly improved COPD patients’ breathlessness from physical activity.
Read the full article at: www.fiercebiotech.com
Continue Reading »Unregulated $7K dental device sparks at least 20 lawsuits
More than 10,000 patients have been fitted with an Anterior Growth Guidance Appliance. But the unproven device has not been evaluated by the FDA.
Read the full article at: www.fiercebiotech.com
Continue Reading »Abbott’s lab-based blood test for concussion scores FDA nod
Two years after the FDA OK’d a handheld test to detect signs of a TBI within 15 minutes, the concussion-spotting test is heading to the big leagues.
Read the full article at: www.fiercebiotech.com
Continue Reading »Blood-Based MRD Testing Identifies AML Patients at Risk of Relapse After Stem Cell Transplant

Researchers looked at AML variants in the blood of patients prior to their allogeneic hematopoietic cell transplant and correlated them with subsequent relapse or death.
Read the full article at: www.precisiononcologynews.com
Continue Reading »