FDA delivers approval to Lumicell’s fluorescent system for highlighting residual breast cancer tissue
The injectable Lumisight agent was approved via the FDA’s drug review pathway, while the visualization system took a parallel track through the agency’s device center.
Read the full article at: www.fiercebiotech.com
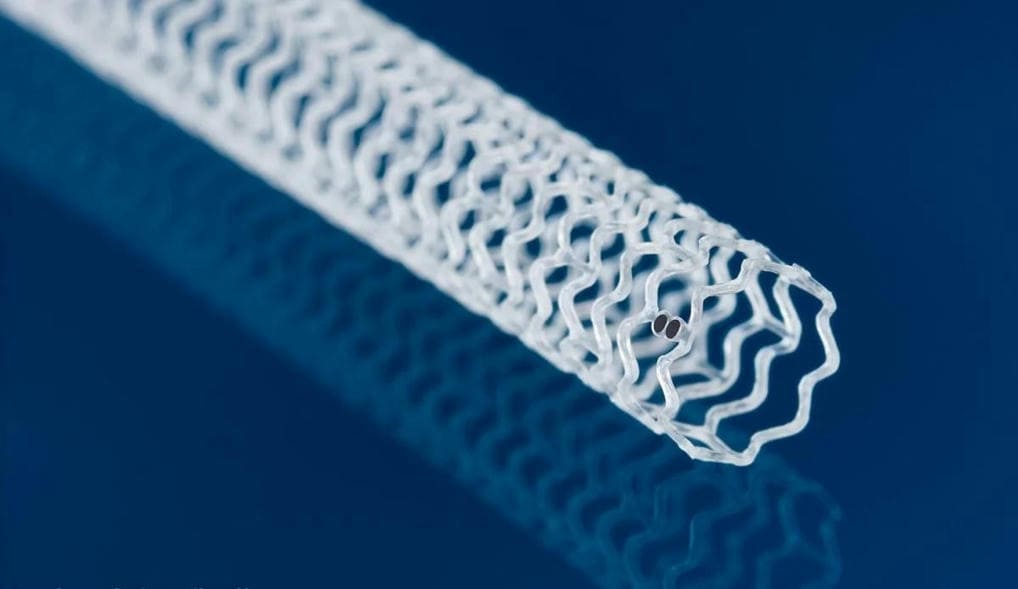
Continue Reading »FDA approves Abbott’s dissolving stent for blocked arteries below the knee

Abbott has returned to the field of bio-absorbable, dissolving stents, and in somewhat uncharted territory. The Esprit BTK implant aims to treat the most severe form of peripheral artery disease, a condition known as chronic limb-threatening ischemia.
Read the full article at: www.fiercebiotech.com
Continue Reading »FDA delivers de novo clearances to two novel antibacterial implant coatings

Though the initial green lights cover spine and orthopedic hardware, Orthobond and Onkos Surgical said their respective disinfectants could shield a wide variety of implants.
Read the full article at: www.fiercebiotech.com
Continue Reading »MMI’s microsurgery robot secures de novo clearance from FDA
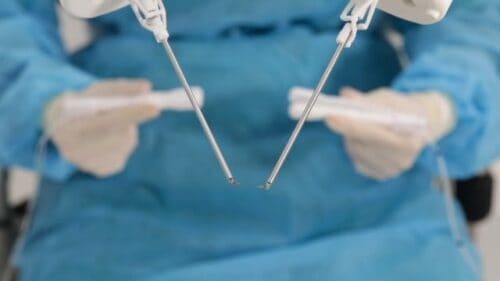
Hot on the heels of a $110 million fundraising, Medical Microinstruments has now received a groundbreaking green light from the FDA for its robotic surgery system.
Read the full article at: www.fiercebiotech.com
Continue Reading »Medtronic snags FDA approval for body-sensing, closed-loop spinal cord stimulator

The Inceptiv implant for chronic pain aims to keep pace with the body’s nerve activity as the patient goes through their daily life.
Read the full article at: www.fiercebiotech.com
Continue Reading »Harnessing AI and Machine Learning in Medical Device Design
Medical device design has evolved significantly, driven by technological advancements and a deeper understanding of patient needs. Integrating artificial intelligence (AI) and machine learning stands out as a transformative development […]
Continue Reading »FDA Clears RedDrop Dx, Inc.’s Blood Collection Device, RedDrop One
FDA Clears RedDrop Dx, Inc.’s Blood Collection Device, RedDrop One – read this article along with other careers information, tips and advice on BioSpace…
Read the full article at: www.biospace.com
Continue Reading »FTC asks Boston Scientific, Axonics for more info on their $3.7B deal, pushing back acquisition timeline

The multibillion-dollar deal for Axonics and its neurostimulation therapies is now slated to close during the second half of this year.
Read the full article at: www.fiercebiotech.com
Continue Reading »Smith+Nephew introduces the RENASYS™ EDGE Negative Pressure Wound Therapy System – an exciting new option in home-based care for patients living with chronic wounds

Smith+Nephew introduces the RENASYS™ EDGE Negative Pressure Wound Therapy System – an exciting new option in home-based care for patients living with chronic wounds – read this article along with other careers information, tips and advice on BioSpace…
Read the full article at: www.biospace.com
Continue Reading »Biden directs agencies to boost R&D for women’s health

After asking Congress to fund women’s health research with $12 billion, President Biden is doing what he can to boost investment via executive order.
Read the full article at: www.fiercebiotech.com
Continue Reading »